Physostigmine salicylate (physostigmine) is a carbamate ester and an indole alkaloid that acts as an acetylcholinesterase inhibitor to enhance the transmission of acetylcholine signals in the brain by crossing the blood-brain barrier to enter and stimulate the central nervous system.
Primarily used as an injection, the primary function of physostigmine salicylate is as an antidote to reverse the effect of drug-induced anticholinergic syndrome.
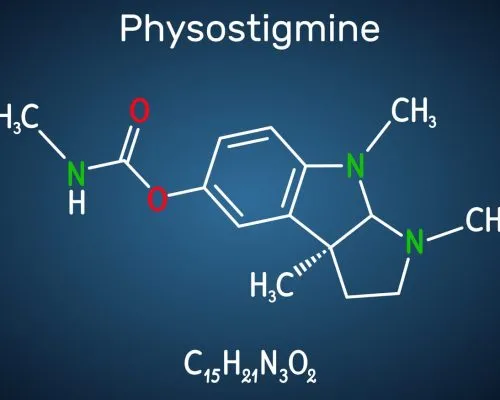
Physostigmine Structure
Physostigmine is a white, odorless, microcrystalline powder with a molecular formula of C15H21N3O2.C7H6O3 or C22H27N3O5. The primary components of Physostigmine Salicylate are CID 5983 (Physostigmine) and CID 338 (Salicylic acid).
Physostigmine Salicylate Medical Uses
Primary medical uses for physostigmine salicylate include:
- Treatment of glaucoma
- Delayed gastric emptying
- Treatment of anticholinergic poisoning caused by overdoses of atropine, scopolamine and other anticholinergic drugs
- Reversal of neuromuscular blocking drugs
- Veterinary use as a cholinergic (anticholinesterase) agent
Get your requested raw materials quotation
Physostigmine Salicylate History
Physostigmine occurs naturally in the seeds of Physostigma venenosum (also called calabar bean or ordeal bean), a plant native to the Old Calabar province in Eastern Nigeria. It was used to detect practitioners of witchcraft by forcing the accused to consume seeds with the belief that they were guilty if they died as a result.
Physostigma venenosum was initially studied by European scientists in the mid-1800s, and clinically introduced the drug for use in 1864 as a cholinesterase inhibitor. German-born chemist Otto Loewi conducted studies in the 1920s that led to the discovery of biochemical transmitters. This led to him receiving the Nobel Prize in Physiology or Medicine in 1936, shortly after the chemical was synthesized in 1935 by chemist Percy Lavon Julian.
Physostigmine was first commercially produced for intravenous or intramuscular injection by O’Neal, Jones and Feldman Pharmaceuticals in the United States. Anesthesiologists initially used physostigmine to reverse the effects of atropine, antidepressants, sedatives, neuromuscular blocking or relaxant agents. The drug was studied as a possible way to improve long-term memory and treat Alzheimer’s disease, however was not shown to offer benefits in clinical trials.
How Physostigmine Salicylate Works
Physostigmine prevents the action of acetylcholinesterase, an enzyme responsible for breaking down acetylcholine. Through the interference of acetylcholine metabolism, physostigmine is responsible for indirectly stimulating both muscarinic and nicotinic receptors due to a significant increase of acetylcholine at the synapse.
Dosage recommendations for physostigmine salicylate are as follows:
Post-Anesthesia Care:
- 0.5-1.0 mg/kg intravenous injection or intramuscular administration
- Slow controlled rate of maximum 1 mg per minute
- Repeat dosage at 10-30 intervals if required
Anticholinergic Drug Overdose
- 2.0 mg/kg intramuscular administration or intravenous injection
- Slow controlled administration recommended
- Repeat dosage recommended in the case of life-threatening signs such as convulsions, arrhythmia or coma
Pediatric dosage
- 0.02 mg/kg by slow intravenous injection or intramuscular administration
- Maximum 0.5 mg/minute
- Repeat dosage recommended at 5-10 minute intervals until desired therapeutic effect obtained
- Maximum 2 mg dosage recommended
Hyaluronidase Side effects
Hyaluronidase may cause some side effects that do not require immediate medical attention. These reactions may gradually disappear as the body adjusts following injection. They include:
- Bleeding
- Blistering
- Burning sensation
- Cold sensations
- Skin discoloration
- Hives
- Feelings of pressure
- Inflammation (hot sensations)
- Infection
- Skin conditions
- Itchy skin
- Lumps
- Rash/redness
- Numb feelings
- Pain/soreness/stinging
- Scars
- Swelling/tenderness/tingling sensation, ulceration, or warmth sensation at the injection site
A medical professional may help mediate or help reduce some of the side effects, and it is advised to consult with a health care professional if they continue.
Side effects of hyaluronidase that require immediate medical attention include:
- Cough
- Swallowing difficulty
- Dizziness
- Increased and/or rapid heartbeat
- Hives or welts on the face, eyelids, lips, tongue, throat, hands, legs, feet, or sex organs (hives, welts or itching)
- Skin redness or rash
- Itching
- Puffiness or swelling of the eyelids, eyes, face, lips, or tongue
- Chest tightness
- Weakness or fatigue
Symptoms of hyaluronidase overdose include:
- Blurry vision
- Body chills
- Confused thinking
- Faintness, dizziness, or lightheadedness when rising suddenly from a sitting or lying position
- Irregular, rapid or pounding heartbeat or pulse
- Nausea
- Vomiting
- Swelling
- Skin redness
- Increased skin warming
- Sweating
- Fatigue, tiredness or weakness
Physostigmine Clinical Trials
The following clinical trials have been conducted for physostigmine:
[NCT Number – Date – Condition – Sponsor]
- NCT04366518 – April 15, 2021 – Hallucinations, Auditory|Psychosis – Yale University
- NCT03090620 – March 30, 2017 – Anticholinergics Toxicity – University of Colorado, Denver|American Academy of Clinical Toxicology
- NCT03013322 – January 2015 – Shock, Septic|Sepsis|Perioperative Period – University Hospital Heidelberg
- NCT02216266 – April 2014 – Suspected Delirium After Elective or Emergency Heart Surgery|CAM-ICU Diagnosed Delirium – PD Dr. Bertram Scheller|University Hospital, Frankfurt|Dr. Franz Köhler Chemie GmbH (study medication and labeling)|Johann Wolfgang Goethe University Hospital
- NCT02008292 – September 2013 – Smoking|Schizophrenia – Yale University|National Institutes of Health (NIH)|National Institute on Drug Abuse (NIDA)
- NCT01394445 – June 2011 – Postoperative Pain – Medical University of Graz
- NCT01121497 – July 2010 – Cognitive Dysfunction – Rabin Medical Center
- NCT00850850 – December 2009 – Postoperative Nausea and Vomiting|Anesthesia Recovery Period – Bispebjerg Hospital
- NCT01171118 – August 2009 – Upper Airway Obstruction – University of Rochester
NCT00689208 – May 2007 – Insulin Resistance – AstraZeneca
Physostigmine Salicylate Contraindications
The use of physostigmine salicylate injections should be avoided in patients experiencing the following conditions:
- Asthma
- Diabetes
- Gangrene
- Cardiovascular disease
Physostigmine salicylate is also contraindicated in cases where there is mechanical obstruction of the urogenital tract, intestine or any vagotonic state. Patients receiving depolarizing neuromuscular blocking agents such as decamethonium or succinylcholine and choline esters should also avoid use of the drug. Additionally, in the case of post-anesthesia, the concomitant use of physostigmine salicylate with atropine is not recommended due to the antagonist action of physostigmine with atropine.
Get your requested raw materials quotation
Physostigmine Salicylate Side effects
Moderate to severe side effects of physostigmine salicylate include:
- Nausea
- Anorexia
- Vomiting
- Diarrhea
- Tremors
- Abdominal pain
Physostigmine Salicylate FAQ
Derived from the seeds of Physostigma venenosum, physostigmine salicylate is a carbamate ester and an indole alkaloid that inhibits acetylcholinesterase to cross the blood-brain barrier and enhance the transmission of acetylcholine signals to stimulate the central nervous system.
Physostigmine is primarily injected for use as an antidote to reverse the anticholinergic syndrome caused by other drugs.
Methyl salicylate is used as an external analgesic in over-the-counter medicines to temporarily relieve muscle and joint pain and minor body aches caused by arthritis, bruises, sprains and strains.
Salicylates are natural chemicals made by plants and can be found in some fruits and vegetables. They are a poisonous defense mechanism used by plants for protection against disease and insects.
Medica Pharma is a trusted provider of physostigmine salicylate. We partner with GMP manufacturers worldwide to source and provide the highest quality chemical products on the market.
Medica Pharma products are GMP-certified
Good Manufacturing Practices (GMPs) protect consumers through guidelines that ensure the safe manufacture of cosmetics, food, beverages, dietary supplements, medical equipment and pharmaceutical products. Manufacturers conforming to GMP guidelines must meet strict standards to ensure their products demonstrate high quality across batches.
All products sold by Medica Pharma are GMP-certified.